Building servces engineering/Psychrometrics
Introduction
Psychrometrics (or Psychrometry)
is the study of air and water vapor mixtures for air conditioning. For this application, air is taken to be a mixture of nitrogen and oxygen with the other gases being small enough so that they can be approximated by more of nitrogen and oxygen without much error. In this psychrometry section, vapor refers to water vapor. For air at normal (atmospheric) pressure, the saturation pressure of vapor is very low. Also, air is far away from its critical point in those conditions. Thus, the air vapor mixture behaves as an ideal gas mixture. If the partial pressure of the vapor is smaller than the saturation pressure for water for that temperature, the mixture is called unsaturated. The amount of moisture in the air vapor mixture is quantified by its humidity.
The absolute humidity ω is the ratio of masses of the vapor and air, i.e., ω = mv/ma. Now, applying ideal gas equation, pV = mRT for water vapor and for air, we have, since the volume and temperature are the same, ω = 0.622 pv/pa. The ratio of specific gas constants (R in preceding equation) of water vapor to air equals 0.622 .
The relative humidity φ is the ratio of the vapor pressure to the saturation vapor pressure at that temperature, i.e., φ = pv/pv,sat.
The saturation ratio is the ratio of the absolute humidity to the absolute humidity at saturation, or, ψ = ω/ωsat. It is easy to see that the saturation ratio is very close to the value of relative humidity.
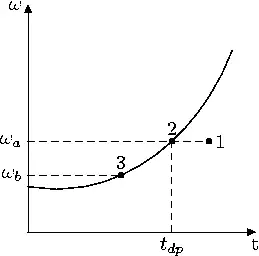
The above plot shows the value of absolute humidity versus the temperature. The initial state of the mixture is 1, and it is cooled isobarically, and at constant absolute humidity. When it reaches 2, it is saturated, and its absolute humidity is ωa. Further cooling causes condensation and the system moves to point 3, where its absolute humidity is ωb. The temperature at 2 is called the dew point.
It is customary to state all quantities in psychrometry per unit mass of dry air. Thus, the amount of air condensed in the above chart when moving from 2 to 3 is ωb − ωa.
Adiabatic Saturation
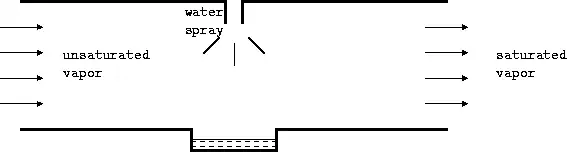
Consider an unsaturated mixture entering a chamber. Suppose water was sprayed into the stream, so that the humidity increases and it leaves as a saturated mixture. This is accompanied by a loss of temperature due to heat being removed from the air which is used for vaporization. If the water supplied is at the temperature of exit of the stream, then there is no heat transfer from the water to the mixture. The final temperature of the mixture is called adiabatic saturation temperature.
Wet Bulb Temperature
The relative humidity of air vapor mixtures is measured by using dry and wet bulb thermometers. The dry bulb thermometer is an ordinary thermometer, while the wet bulb thermometer has its bulb covered by a moist wick. When the mixture flows past the two thermometers, the dry bulb thermometer shows the temperature of the stream, while water evaporates from the wick and its temperature falls. This temperature is very close to the adiabatic saturation temperature if we neglect the heat transfer due to convection.
Psychrometric Chart
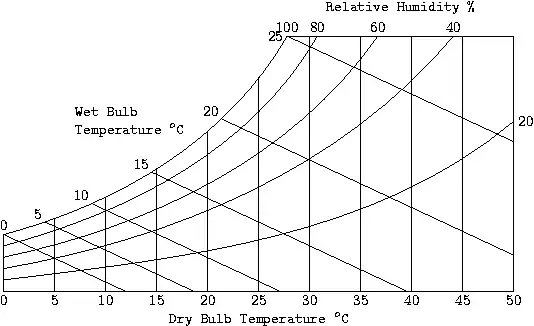
This chart gives the value of absolute humidity versus temperature, along with the enthalpy. From this chart you can determine the relative humidity given the dry and wet bulb temperatures. We have, from the first law, that for a flow system with no heat transfer, the enthalpy is a constant. Now, for the adiabatic saturation process, there is no heat transfer taking place, so that the adiabatic saturation lines are the same as the wet bulb temperature and the constant enthalpy lines.
Questions
1. The temperature at Phoenix is 35 °C with a relative humidity of 40%. Can a room be cooled using a conventional air cooler?
- We need to find the wet bulb temperature for the point T = 35°C and φ = 40%. We have, from the psychrometric chart, the wet bulb temperature is between 20 and 25°C. Thus, you can cool the room down to a comfortable temperature using an evaporative cooler.
2. The temperature of Los Angeles is 37 °C with relative humidity of 83%. To what temperature can a room be cooled using a conventional air cooler?
- The wet bulb temperature is about 34.2°C for this situation. Thus, you cannot use an ordinary cooler to reduce room temperature in this situation. You will need to use an air conditioner.
Air Conditioning
The human body can work efficiently only in a narrow range of conditions. Further, it rejects about 60 W of heat continuously into the surroundings, and more during heavy exercise. The temperature of the body is maintained by the evaporation of sweat from the body. Thus, for comfort, both the temperature and the relative humidity should be low.
Conventional air conditioning consists of setting the humidity at an acceptable level, while reducing the temperature. Reducing the humidity to zero is not the ideal objective. For instance, low humidity leads to issues like high chances of static electricity building up, leading to damage of sensitive electronic equipment. A humidity level of 50% is more acceptable in this case.
The most common method of reducing humidity is to cool the air using a conventional air conditioner working on a reversed Carnot cycle. The vapor that condenses is removed. Now, the air that is produced is very cold, and needs to be heated back up to room temperature before it is released back to the air conditioned area.